SABIC’s Specialty materials, available in Ireland through their distribution partner Ultrapolymers, offer a wide range of potential solutions for healthcare including structural and functional biocompatible materials, FDA food compliant colour packages and sustainable options with renewable content. We support our materials with value-added services, including design support, mold-flow analysis, testing and predictive engineering capabilities, which are available for each step of your application development process.
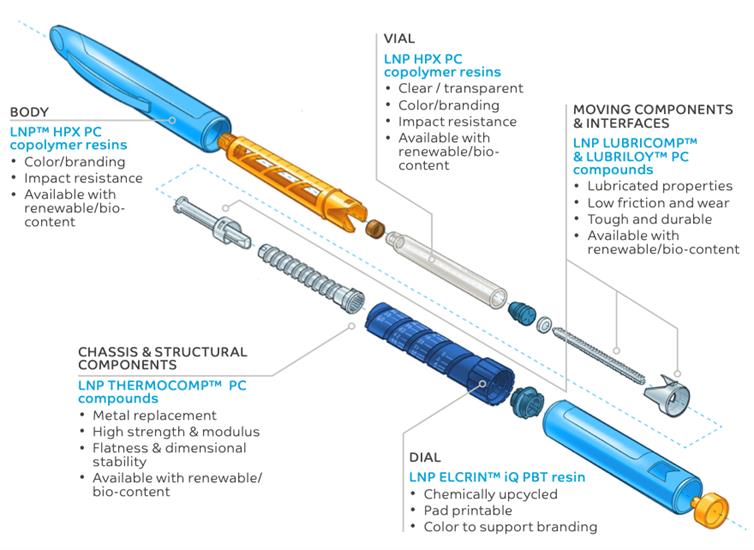
Renewable copolymers compared with conventional copolymers:

To discover how SABIC can help you, please contact SABIC’s local Irish distributor, Ultrapolymers using ask.healthcare@ultrapolymers.com or visit the website www.ultrapolymers.com.